ACCELERATE's 15th Pediatric Cancer Strategy Forum
This year’s focus was on antibody drug conjugates (ADCs) equipped us to be more savvy advocates for pediatric cancer research.
–Robyn Spoon, PhD, Elevate Founder and CEO

Elevate’s mission to save and improve the lives of those diagnosed with pediatric cancer has sent us, and many of our advocates, around the world to learn, advocate and collaborate. ACCELERATE’s 15th Pediatric Strategy Forum focus on antibody drug conjugates (ADCs) equipped us to be more savvy advocates for pediatric cancer research. ACCELERATE embodies a core Elevate value that in order to move the needle towards precision medicine for children on day one of diagnosis requires all of the stakeholders to come together. This strategy forum was attended by a veritable “who’s who” in the pediatric cancer space, including oncologists from COG and abroad, scientists and oncologists from leading pediatric cancer institutes from the USA and abroad, pharmaceutical partners, and advocates. During this forum, this multi-stakeholder forum addressed some of the key problems that have caused the 6.5 year average lag between when drugs are developed/approved for adults and when they become available for children.
The goal of the strategy forum was to create a strategy for the development and prioritization of ADCs for the treatment of pediatric solid malignancies.
- Ahead of the strategy forum, participating advocates received training in the drug class, antibody drug conjugates. This allowed me to be a more useful advocate and that knowledge has already been brought back to advocates within our Elevate community. This knowledge will also directly inform Elevate’s decisions regarding future investments in science.
2. During the strategy forum, every stakeholder present was asked to consider three key questions as we worked to evaluate the use of this drug class for solid tumors in children (Is the target relevant in pediatric cancers? Is the payload relevant? Does the ADC address an unmet need in pediatric cancer?)
Identifying and defining these pinchpoints is allowing us to strategically invest our time and money to ensure children have access to these lifesaving treatments more quickly. This strategy forum was particularly relevant because Elevate collaborated with Winking Warrior Foundation earlier this year to invest in Dr. Lou Stancato’s (IBRI) development of a monoclonal antibody (mAb) to investigate its relevance in metastatic pediatric sarcomas (e.g. rhabdomyosarcoma, osteosarcoma). Not all mAbs become ADCs, but all ADCs include an antibody. This session was directly related to our current science investment! [Learn more about Lou’s work and vision here.]
This strategy forum provided us with a strong overview of the ADCs that pharmaceutical companies are currently considering for trials in a range of solid tumors, including the pediatric cancers Elevate focuses on: sarcoma and pediatric renal tumors. We gained a more holistic overview of the ADCs in the pipeline for pediatric cancer! In addition, as a member of the advocacy core, I had the opportunity to vocalize the needs and concerns from our advocates. In particular, the advocates present had the chance to address the risks versus rewards of different trial designs and inclusion criteria.
Several pharmaceutical companies were there to share updates on their plans to expand the use of ADCs for adult oncology into pediatric trials, including: AbbVie, Daiichi, Day One Pharmaceuticals, Eli Lilly, and Merck. In addition, academic researchers from a host of academic institutions also shared their ADC updates, such as Emily Slotkin, MSKCC. It was exciting to see that several of these investigators are working on expanding trials to include pediatric sarcomas (e.g. rhabdomyosarcoma, osteosarcoma, Ewing sarcoma) and pediatric renal cancers (e.g. Wilms tumor, malignant rhabdoid tumors). Here is an example of an ADC clinical trial for rhabdomyosarcoma: https://clinicaltrials.gov/study/NCT06941272.

My first experience with ACCELERATE was in the spring of 2024, when I traveled to Brussels for the annual event that brings together stakeholders from across the world and across disciplines. I was quickly introduced and given the opportunity to develop relationships with key advocates, such as Donna Ludwinski (Solving Kids Cancer) and Nicole Scobie (ZOE4Life). This recent strategy forum in Denver introduced me to Joan Slaughter and Robin French (The Morgan Adams Foundation), Joe McDonough (B+), Vickie Buenger (CAC2 and longtime childhood cancer advocate), and Susan Weiner (Children’s Cancer Cause) among others. Collaboration is absolutely necessary to achieve our goals. Each of these advocates brings with them a wealth of knowledge, personal experience and connections that has enriched my understanding of this complicated problem. Events such as this ensure that the Elevate community stays up to date on the latest in drug development, including the barriers that we need to advocate to overcome.
—————————————————
Antibody drug conjugates (ADCs):
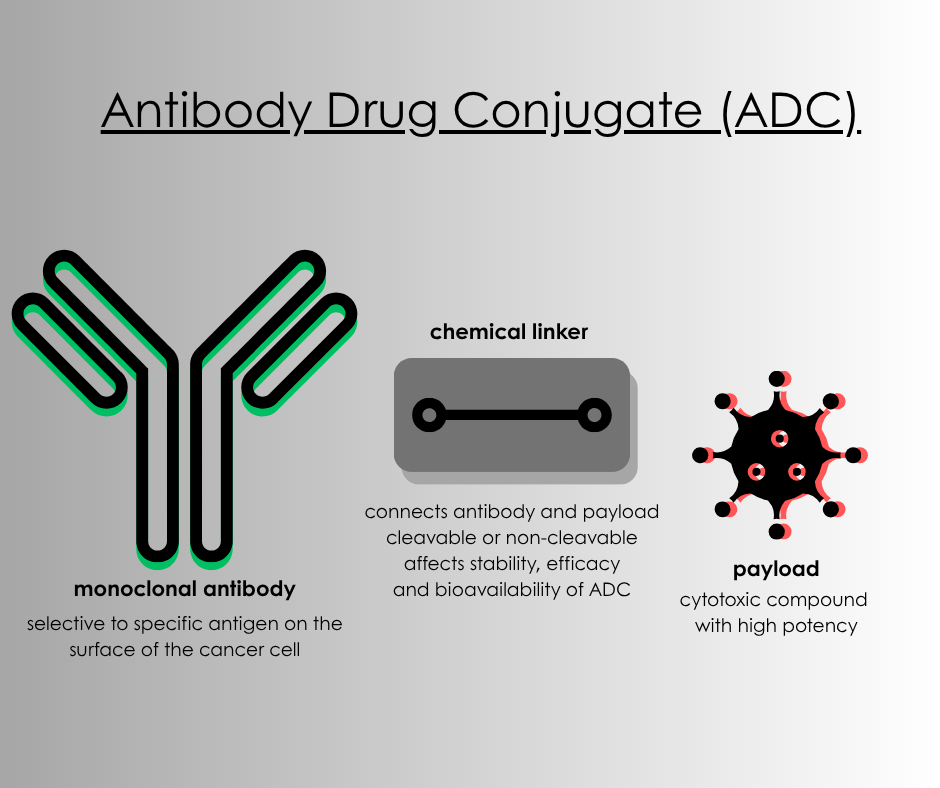
Think of an antibody drug conjugate as precision chemotherapy. At the most basic level, these drugs contain an antibody, a linker and a payload (see Image 1). The goal for the drug maker is to create a monoclonal antibody that is selective to specific antigens on the cancer cells, but is not typically expressed in normal healthy tissue. We also learned that not all linkers are created equal, some are cleavable and others are non-cleavable, which affects the stability, efficiency and bioavailability of the ADC. Some pharmaceutical companies are developing proprietary linkers to improve the effectiveness of the ADC. Finally, a payload containing a cytotoxic compound is integrated into the drug with a goal to destroy the cancer cells. The majority of drug-makers are using a topoisomerase I inhibitor, such as irinotecan, as the payload. ADCs have been used effectively in adult cancers for a long time and have become a drug of heightened interest among industry drug-makers.

————————–


