Advocating at the FDA
ELEVATE at the FDA
“
“Making more drugs available to kids with cancer means solving a million small problems. Yesterday’s event provided a necessary opportunity to bring more and more of those problems to light. If we can identify the problems, name the problems, then we can solve the problems, one at a time!”
– Robyn Spoon, Elevate CEO & Founder


“Faster. Please,” Ann Graham said on behalf of parents in her organization, MIBAgents, during her remarks at the FDA. Sitting next to Ann as a panelist at this event, I wanted to jump out of my chair and cheer, “Yes!”

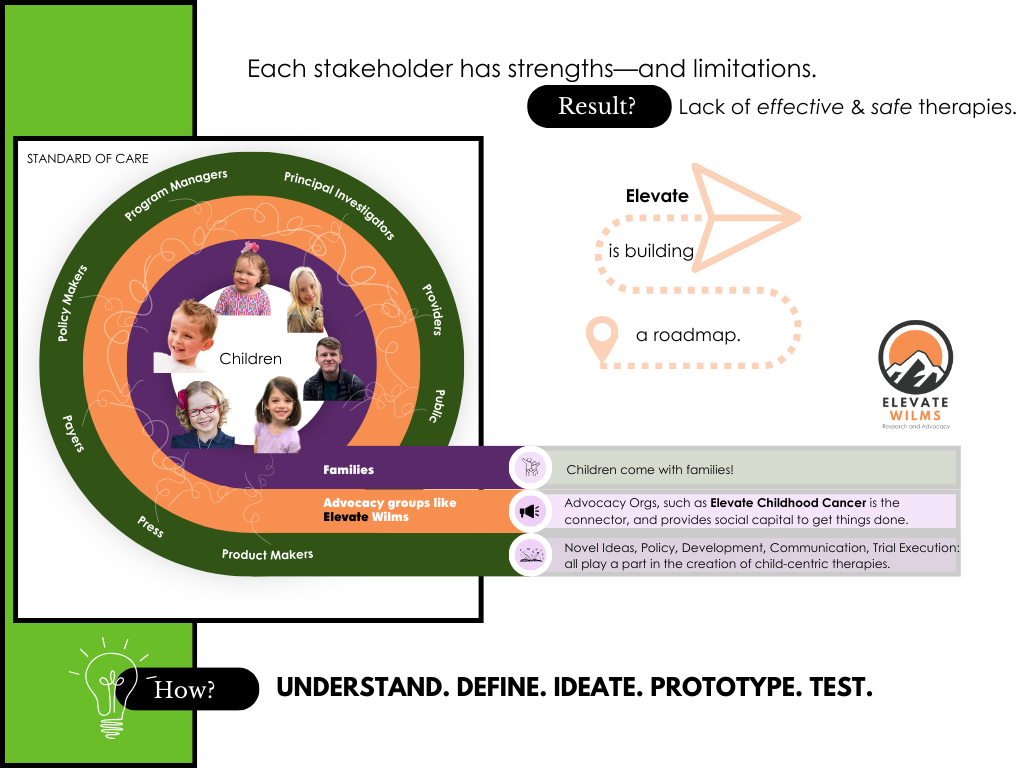
I wake up every morning in a hurry to make the differences our kids have been waiting on for decades. Improvements cannot come fast enough. I am driven by the fact that in the US about 50 families with kids under 18 will find out that their child has cancer every single day, and their medical teams are only as good as the therapies available. There are about 100 different types of childhood cancer, yet most kids are treated with a combination cocktail from a limited stock of chemotherapy agents, rather than with a medication made to directly target their type of cancer. Most of the treatments kids receive were designed for adults and repurposed for kids. These kids are not just numbers to us, they are real children and real families who are facing this. [Meet our Elevate kids and families HERE.]
This is not acceptable—our kids NEED medications designed to treat their cancer.
It turns out developing new drugs for our children is a VERY LARGE and COMPLICATED problem. It reminds me of that saying about “eating an elephant one bite at a time.” Making more drugs available to kids with cancer means solving a million small problems. Yesterday’s event provided a necessary opportunity to bring more and more of those problems to light. If we can identify the problems, name the problems, then we can solve the problems, one at a time!
I suspect it is possible that at first glance many would assume the FDA plays the part of “roadblock” rather than as a partner in therapy development. I’ve had the chance to interact with Dr. Martha Donaghue, Associate Director for Pediatric Oncology and Rare Cancers at the Oncology Center of Excellence several times this year. My first encounter with Martha was as a listener during a day-long webinar about the role of public-private partnerships in pediatric cancer drug development, but during ACCELERATE in Belgium I had the chance to meet her in person. Martha began her career as a pediatrician and then completed a fellowship in pediatric hematology and oncology before assuming her role at the FDA; she brings her lived experiences working with patients to her role. When we met again in New York at Sloan Kettering at the 12th Annual Pediatric Renal Tumor Biology Summit this summer she invited me to attend this event. Martha is a champion for advocates as a critical stakeholder in the development of new therapies for kids with cancer!
[Read more HERE from Elevate’s involvement at the Summit.]
I took some of my own learnings from committee work at ACCELERATE, my time at Sloan Kettering, countless meetings with scientists, oncologists, and families to share a few thoughts about making trial enrollment easier for families with the leaders at the FDA. Our personal experience traveling over a thousand miles from home to participate in the one available and open trial is the “norm” for families! Discussing ways to decentralize aspects of trials and making these trials available in more centers across the country is needed to reduce the burdens families face when they are already buried in exhaustion and financial stress. [Learn more HERE about what a clinical trial is.]

Nancy Goodman (Kids v Cancer), architect and advocate of life-changing legislation on behalf of kids with cancer, shared that Martha and her team at the FDA are “superheroes.” After spending the day at the FDA, I can say that the combined talent and skill set of those who spoke is insane. Biostatisticians, oncologists, pharmacologists, biologists: all working together to help make sure new drugs or repurposed drugs are effective and safe for our kids!
The message I hear over-and over again from the regulators is to invite them in as early as possible!
I heard the message loud and clear, and it will inform Elevate’s investments in science and the way we advocate in this space. Those of us investing in scientific efforts need to ask who on their team has regulatory know-how and when they plan to bring the FDA into their process, otherwise, amazing and novel advancements in research labs will get stuck.
I always come prepared with some goal our organization has in mind. In this case, my goal was to learn more about the potential benefits of a natural history study or patient registry, things our Elevate workgroups [HERE] have been discussing for some time now. We’ve seen other cancer types, like breast cancer, do this to help make faster changes in available therapies. Making it easier for drug makers to study the effects of their product and giving as many kids as possible the chance to try that drug is a top priority. As Ginny (Swifty Foundation/ Gift from a Child) shared in her plea to the FDA, kids with a known terminal illness shouldn’t have to risk receiving a placebo when they participate in a drug trial. While there are many possible solutions to this problem, having a patient registry or natural history study with good quality data for regulators to use as a comparison or external control, is one possibility!
Each event needs to springboard the next steps Elevate takes to bring innovative and life-changing therapies to those who need them most: our kids! So, my morning has been spent following up with new and old partners identifying the next priorities to keep moving this behemoth of a machine forward ever more quickly!!
It was also helpful to get an update directly from Nancy Goodman regarding legislative efforts to make developing drugs for kids with cancer easier! Connecting the dots were members of the FDA staff that provided details of the four newly FDA approved drugs this year alone, and which specific pieces of legislation supported their approval process. Armed with that knowledge, I headed to Capitol Hill to discuss the Give Kids a Chance Act and Creating Hope Reauthorization Act.

If you’ve made it to the bottom of this article, thank you! You’re likely interested in learning how you can make a real difference.
Are you ready to become an informed and savvy advocate?
Join us as we dramatically improve and save the lives of those diagnosed with childhood cancer, start by reaching out today! Not sure how your skills can make an impact? Let’s talk–we’ll find a way for you to get involved. We’ve got a lot of work to do and need your help!
Empowerment. Advocacy. Progress.

Want to stay informed about Elevate’s progress?
Sign up for our quarterly newsletter below to keep up with our efforts.
Your donations, whether one-time or recurring, directly fuel our mission, to let kids be kids as we dramatically improve and save the lives of those diagnosed with childhood cancer.

@2023 ELEVATE Childhood Cancer Research
and Advocacy, Inc.
is a registered 501(c)(3) non-profit organization.
EIN: 93-2185372
Join us! Stay up to date with our work and mission, sign up for Elevate's newsletter!
Please help keep our site secure and solve this problem.
We respect your privacy and will never sell or share your information.